REVERSE OSMOSIS
What is the reverse osmosis?
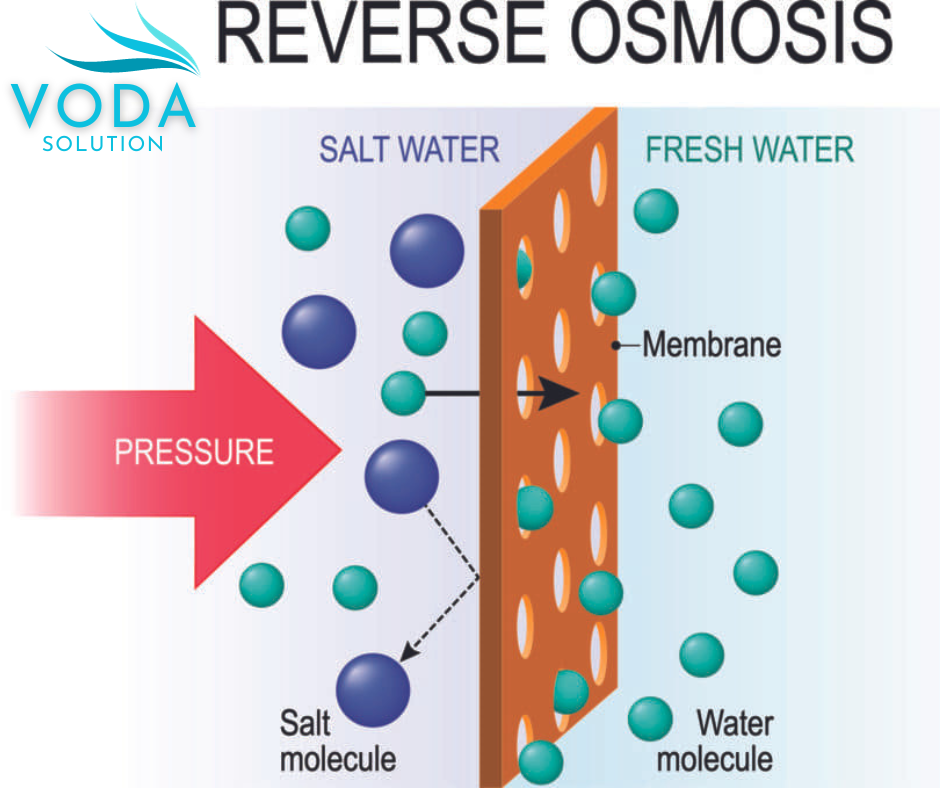
Reverse osmosis (RO) is a process in which the flow rate through a semipermeable membrane is reduced,
a thrust force greater than the osmotic pressure is exerted in the opposite direction to the osmosis process (Figure 1).
It is possible to separate the substance found in the water on one side of the membrane and on the other side a dilute solution low in dissolved solids (permeate) is obtained.
It could be said that it is a physical filter but because it is a membrane that retains solid elements,
but we can no longer call it filtration because it retains dissolved molecules that are not only retained by physics,
but also by chemistry.
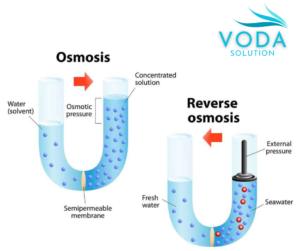
Figure 1. Osmosis and reverse osmosis process diagram.
Reverse osmosis is used to desalinate seawater and brackish water, soften water, remove organic matter, and separate specific contaminants from water.
What is osmosis?
Osmosis is the process by which a solvent passes through a semipermeable membrane,
from a dilute solution to a concentrated one, until the difference in concentrations on both sides of the membrane is equal.
The pressure required for this phenomenon to occur is known as osmotic pressure.
How does a reverse osmosis plant work?
Reverse osmosis plants require pretreatment systems, feeding pumping equipment, pressurized tanks (membrane holders or housings)
that contain the membranes, chemical dosing equipment, etc. for them to work properly.
- Membrane:This element is made by rolling membranes into a spiral, usually measuring 40 or 60 inches long, and the most common diameters are 4 or 8 inches. During operation, water enters under pressure on one side of the housing, as it flows tangentially to the membrane, part of it passes through the surface of the membrane towards the permeate collector, while water with a high concentration of salts exit through the other end of the membrane.
- In Figure 2 you can see the elements of a membrane.
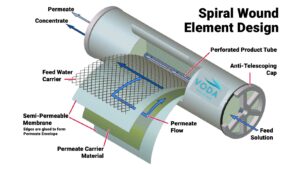
Figure 2. Elements of an RO membrane
What are the types of reverse osmosis membranes?
Reverse osmosis membranes are the most common types for purification of drinking water, brackish water and seawater.
Osmosis membranes for brackish water
Brackish water is fresh and salt water mixed together. Therefore it is saltier than fresh water, but not as salty as seawater.
These waters are very common in aquifers near the sea, or can be found in brackish fossils.
Brackish water total dissolved solids (ppm): Brackish: 1,000 – 2,000 parts per million
Fresh water: <500
Slightly brackish: 500 – 1,000
Moderately saline: 2,000 – 5,000
Salt water: 5,000 – 10,000
Sea water: 10,000 – 35,000
Brine: >35,000
Reverse osmosis membranes for brackish water
| brackish water membranes | seawater membranes | High rejection membranes | Low energy membranes | Membranes under fouling |
| 2.5” | 2.5” | 2.5” | 2.5” | 2.5” |
| 4″ | 4″ | 4″ | 4″ | 4″ |
| 8” | 8” | 8” | 8” | 8” |
* Consult for other needs
These elements can be installed in different arrangements, the concentrate from one membrane can feed another to increase water recovery.
Another arrangement is to feed one membrane with the permeate from another,
with the aim of further reducing the concentration of dissolved solids in the water.
Reverse osmosis pre-treatment
- Pre-treatment: Pre-treatment of reverse osmosis systems is important to extend the life of the membranes and obtain better performance in reducing dissolved solids.
One of the purposes of pretreatment is to prevent scale.
This phenomenon generally occurs when low-solubility salts, such as calcium and magnesium, deposit and become embedded in the pores of the membranes.
Scale control consists of adjusting the pH (modifies the solubility of these salts) or adding antiscalant (prevents the formation of crystals or slows their growth).
Other contaminants that can affect RO membranes are suspended solids, these can clog the feed or saturate the surface of the membrane.
A pre-treatment process for this issue is filtration.
It is recommended to use filter that retain all substances bigger than 5 microns.
Typically 5 micron absolute or nominal 1 micron cartridge filters are used.
Disinfection is another typical pretreatment step used to prevent biological saturation of the membrane.
It is extremely important to verify that the membrane material and disinfectant agent are compatible,
because many of these can permanently damage the osmosis membrane.
Classification separation membranes (the opening of their pores)
- Microfiltration: 0.1 – 1 microns (μm)
- Ultrafiltration: 0.01 – 0.1 μm
- Nanofiltration: 0.001 – 0.01 μm
- Reverse osmosis (hyperfiltration): 0.0001 – 0.001 μm
Remember that:
1mm = 1000μm
1 μm = 1000 nanometers (nm)
1 nm = 10 Angstroms (Å)
It is impossible to overstate the significance of membrane technology advancement for day-to-day living.
Membrane technology has gained a lot of popularity in recent decades for producing potable water for human use.
However, the water treatment industries still use a lot of outdated, energy-intensive technology like ultrafiltration and reverse osmosis.
This paper examines the low energy consumption of membrane distillation (MD) technology,
focusing on the development of materials and energy requirements.
The history of the MD process, configurations, module kinds, membrane materials, fabrication processes, properties,
methods of material modification, applications, and energy requirements are all covered first.
Then, a thorough discussion of the design and operating condition factors that affect MD performance follows.
Moreover, we note that the two most popular applications for MD that have attracted the most attention are desalination and wastewater treatment.
Improvement in MD performance is being pursued in two crucial areas: membrane hydrophobicity and fouling resistance.
Next, we go over the many sectors in which MD is currently being used.
We highlight the energy needs of MD technology in our conclusion, and we identify solar energy as a renewable energy source that may be able to supply those needs.
This review paper aims to stimulate interest in and offer insights into material development for applications related to membrane distillation.
The choice of membrane materials is one of the challenges in the development of MD.
For MD to handle the operating principle and performance, membrane materials must have certain qualities.
The membranes used in the MD process should exhibit good thermal stability, be hydrophobic, porous, non-dampened, and have low thermal conductivity at high temperatures.
Membrane materials are categorized using a number of features. Materials for membranes might be natural or manmade.
Natural membrane materials, such as polymers like polysaccharides and rubbers, are frequently found in plants and animals.
Membranes can be divided into three categories: mixed-matrix, which comprises both organic and inorganic materials, and organic,
which includes polymers, and inorganic, which includes ceramic, metals, zeolites, and carbon.